Abstract
Introduction: Allogeneic hematopoietic stem cell transplantation (HSCT) remains one of the only curative therapies for acute myeloid leukemia (AML) in adult patients. However, a high proportion of patients will relapse post-HSCT. Measurable residual disease (MRD) has emerged as highly predictive of relapse after chemotherapy and multiple studies have demonstrated inferior outcomes after HSCT for patients with pre-HSCT MRD. Fewer studies have evaluated post-HSCT MRD, but those have suggested that MRD is a powerful predictive tool in this setting as well. We have previously utilized droplet digital PCR (ddPCR) as an MRD platform to track 33 unique AML-associated mutations in a cohort of patients receiving either cytotoxic chemotherapy or targeted agents, and our preliminary data suggest that persistence or recurrence of these mutations during therapy is predictive of relapse and diminished overall survival. In the present study we sought to evaluate the utility of ddPCR specifically for post-HSCT MRD monitoring.
Methods: Patients 18 years of age or older with AML who had received allogeneic HSCT were identified via retrospective chart review. Thirty-seven patients were identified who had sufficient diagnostic testing to identify AML-associated point mutations and had available DNA from serial bone marrow samples. Thirty-six of these patients had at least one pre-HSCT sample analyzed; post-HSCT day 28, day 80, and day 365 samples were available for 26, 25, and 11 patients, respectively. DdPCR was utilized to track 21 different AML-associated mutations in this patient cohort. MRD negativity was defined as variant allelic frequency (VAF) below the limit of detection for all assayed mutations (1-4 mutations per patient). Relapse-free (RFS) and overall (OS) survival were defined as time from date of transplant to relapse or death. Survival statistics were analyzed as Kaplan-Meyer curves, with p-values determined by the log-rank (Mantel-Cox) test. P-values less than 0.05 were considered statistically significant. Outcome data for all patients were censored July 6, 2018.
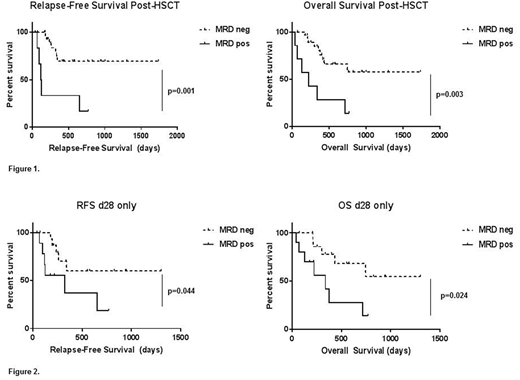
Results: Median follow-up from time of HSCT was 13.3 months. Of the 37 patients included in this cohort, the majority (73%) received regimens containing cytotoxic chemotherapy prior to HSCT; the remainder (27%) received targeted therapies +/- low-intensity chemotherapy prior to HSCT. Only 6 of 36 patients (17%) were MRD negative pre-HSCT, all of whom had received cytotoxic chemotherapy. All 37 patients had one or more post-HSCT samples available for MRD assessment. Of these, 30 achieved MRD negativity after HSCT (81%). Post-HSCT MRD status was predictive of both RFS and OS (Figure 1). This replicates previously published data with respect to ddPCR-based MRD, but is based on evaluation of both more patients and more mutations. In agreement with previously published data showing high predictive value of the 1-month post-HSCT MRD, we found this timepoint specifically to be predictive of RFS and OS in the 26 patients for whom a sample was assayable (Figure 2). Neither outcomes nor MRD status by ddPCR varied based on conditioning regimen (myeloablative versus reduced-intensity) in our cohort (data not shown).
We also compared ddPCR MRD status with whole bone marrow chimerism measured by short tandem repeat (STR) analysis. Concordance between MRD negativity and >95% donor chimerism was 69% at 1 month post-HSCT and 93% at 80 days post-HSCT. Of 8 discordant samples at the 1 month post-HSCT time point, 3 had donor chimerism <95% but were MRD negative, with no relapses to date. The other five patients had full donor chimerism with positive MRD, and 3 of 5 patients have relapsed. Of two discordant samples at 80 days post-HSCT, both involved full donor chimerism with persistence of MRD, and one patient has subsequently relapsed.
Conclusions: ddPCR-based molecular MRD assessment is predictive of relapse-free and overall survival post-HSCT in patients with AML, and may be a powerful adjunct to surveillance measures already in clinical use, such as chimerism. Presence of molecular MRD specifically at 1 month post-transplant showed significant correlation with relapse and with overall mortality. Detection of AML-associated mutations at this and later time points in a prospective manner could in many cases allow salvage of patients prior to clinical relapse, thus improving survival for this high-risk population.
Pollyea:Gilead: Consultancy; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Curis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Celyad: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal